Fill in the blanks(5 marks)
- The molecular formula of Oxygen is ____________
- Percentage Composition of carbon dioxide in air is ___________
- In preparation of oxygen the catalyst used is ____________
- Non metallic oxides are ___________in nature
- CxHy+O2———à_________+__________
Answer in brief (10 marks)
- State how oxygen occurs in free state and combined state.
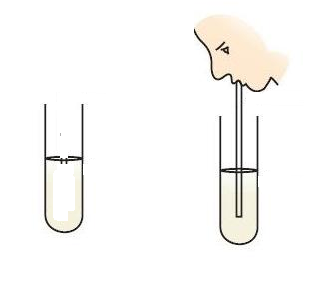
- The solution in the test tube turns milky after blowing air from mouth.Name the solution in the test tube.Write the conclusion of the experiment
3. Write an experiment to presence of water contents in air, with diagram
4.Write the product and chemical equation
- Respiration
- Rusting
5.Draw a neat labeled diagram to show oxygen is noncombustible but supporter of combustion
Answer in detail
- Explain Lavoisier’s experiment with observations and conclusions
- How do you prove any magnesium gains weight after burning?
- Write three justification to say that air is mixture
- When is acidic oxides formed? Give examples of acidic oxides? Write the chemical equations when acidic oxides are dissolved in water
- Write uses of oxygen
