- Take 3 g of Barium Hydroxide in a test tube and now add about 2g of ammonium chloride and mix the contents with the help of a glass rod. Now touch the test tube from outside
- What do you feel on touching the test tube
- State the inference about the type of reaction occurred
- Write the balanced chemical equation of the reaction involved (3 marks, SA1 2016-17)
- Two aluminium strips were kept immersed in ferrous sulphate solution taken in a test tube. The change which was observed is :
- a colourless gas with the smell of burning sulphur is released
- Light green solution changes to blue
- The green solution slowly turns to brown
- The lower end of the test-tube becomes slightly warm (SA1 2016-17)
- Write balanced equations for the following reactions and identify the type of reaction in each case
- Silver Nitrate (aq) +Potassium Iodide (aq)—à Silver Iodide (s) + Potassium Nitrate (aq)
- Potassium Chlorate (s)———-àPotassium Chloride (s)+ Oxygen (g) (SA1 2015, 3 marks)
- A few small pieces of aluminium metal were added to ferrous sulphate solution. It was observed that:
- Pale green colour solution disappears and it becomes colourless
- Pale green colour persists
- Pale green colour of solution turns blue
- Pale green colour of solution turns red (SA1 -2015)
- 2Al+3CuSO4—–à 3Cu + Al2(SO4)3
The type of reaction shown above and the change of colour of reaction solution to product that was observed is
- Combination reaction , blue to green
- Displacement reaction blue to colourless
- Decomposition reaction , blue to green
- Displacement reaction, blue to green
- Consider the following reaction
6. Consider the following reaction
Pb(NO3)2(s)—————-à PbO(s)+NO2(g)+O2(g)
- Write the name and colour of the NO2 gas formed
- Balance the above chemical equation
- Name the type of chemical reaction (SA1-2016-17, 3 marks)
7. a. Can a displacement reaction be a redox reaction? Explain with the help of examples
b. Write the type of chemical reaction in the following: (SA1 2016-17, 5 marks)
i) Reaction between an acid and a base
ii) Rusting of iron
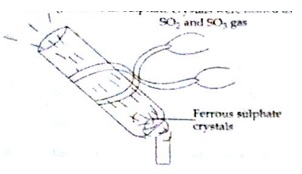
- About 2.5 g of ferrous sulphate crystal were heated as show in the figure below(SA1-2016-17- 2 marks)
- What change in colour of ferrous sulphate crystals would you observe
- On smelling the gases carefully, what would you feel?
9. A) balance the following chemical equations(SA1-2016-17- 5 marks)
- NaOH+H2SO4—-àNa2SO4+H2O
- PbO+C—àPb+CO2
- Fe2O3+Al—-àAl2O3+Fe+Heat
B) Write the balanced chemical equations for the following reactions
- Barium Chloride+Potassium sulphate—à Barium Sulphate+Potassium Chloride
- Zinc+ Silver nitrate–àZinc Nitrate +Silver
10. If M+BX—àMX+B and B is seen as reddish brown deposit, then M and BX are(SA1-2016-17)
- Iron and aluminium sulphate
- Iron and zinc sulphate
- Aluminium and copper sulphate
- Zinc and Iron aluminium sulphate
11. Color of Al2(SO4)3 is (SA1-2016-17)
- Green
- Yellow
- Blue
- Colourless
12. Give chemical equations for the following reactions (5 marks)
- Digestion of food in our body
- Rusting of iron
- Heating of manganese dioxide with aluminium powder
- Blue colour of copper sulphate solution disappears when iron fillings are added to it
- Dilute hydrochloric acid added to sodium hydroxide solution to form sodium chloride and water
