History of finding the smallest particle of a matter:
- Indian and Greek philosophers tried to find which is the smallest particles of matter
- Around 500 BC, Indian Philosopher Kanaada postulated if we go on dividing matter, ultimately we shall come across the smallest particles, beyond which further division is not possible. The particles were named as “Paramanu” by him
- Another Indian philosopher Pakudha Katyayama, said these particles normally exists in a combined form
- Ancient Greek philosophers- Democritus and Leucippus suggested that if we go on dividing matter, a matter, a stage will come when the particles obtained cannot be divided further.
- Democritus called these particles as atoms –meaning indivisible
- At the end of 18th century scientists recognized difference between elements and compounds
Laws of Chemical combination:
-
Law of conservation of mass
Mass can be neither be created nor destroyed in chemical combination
X + Y——————à Z
Suppose, mass of reactant X is m grams and mass of reactant Y is n grams, the mass of product Z will be (m+n) grams
-
Law of constant proportion
In a chemical substance the elements are always present in definite proportion
Eg: In water , the ratio of mass of hydrogen and mass of oxygen is always 1:8. That is if 9 grams of pure water is decomposed, 1 g of hydrogen and 8g of oxygen are always produced
In ammonia(NH3), mass of nitrogen and mass of hydrogen are in the ratio 14:3
In approach to give explanation to these laws, Jhon Dalton provided basic theory about nature of matter.
Dalton’s postulates:
- All matters are made up of very tiny particles called atoms
- Atoms are indivisible particles, which cannot be created or destroyed in chemical reaction
- Atoms of a given element are identical in mass and chemical properties
- Atoms of different elements have different masses and chemical properties
- Atoms combine in the ratio of small whole numbers to form compounds
- The relative number and kinds of atoms are constant in a given compound
Atoms:
What are atoms?:
Atoms are building blocks of any matter
What is the size of an atom?
Atoms are very small, cannot be seen with naked eye. Atomic radius is measure in nanometers.
1nm=1/ 109 m
1m= 109 nm
Symbols of atoms:
Dalton was the first scientist to use symbols for elements. Each symbol meant definite quantity of that element ie. one atom of the element.

Berzillius suggested the symbols of elements to be made from one or two letters of the name of the element.
How the symbols of elements are formed?
- The first letter of the name is symbols for some elements
Eg: Hydrogen H
Oxygen O
Carbon C
- In some cases 2 elements have same first letter. In that case, the symbols are formed by first and second letter of the name. First letter is written in capital and the second in the smaller case letter.
Eg:
Cobalt- Co
Aluminum –Al
- Some symbols are formed by first letter and a letter appearing later in the name.
Eg:
Chlorine- Cl
Zinc-Zn
- Other symbols have ben taken from the names of elements in Latin, German and Greek
Eg:
Fe—From Latin name Ferrum for iron
Na—From natrum for sodium
K- From Kalium for Potassium
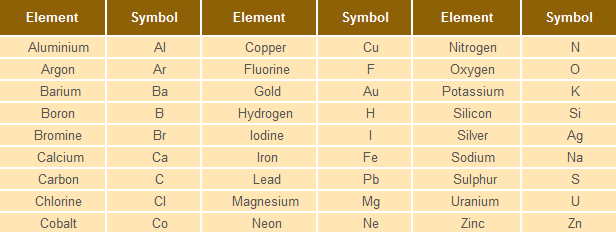
Some elements and their symbols

Atomic mass:
Determining the mass of an individual atom was relatively difficult task. So relative atomic masses were determined using laws of chemical combination
Atomic mass unit:
Earlier scientists took 1/16 mass of an atom of naturally occurring Oxygen as the amu.
This was relevant for the following reasons:
- Oxygen reacted with large numer of elements and compounds
- This atomic mass unit gave masses of most of the elements as whole number
In 1961 ,for universally accepted amu,Carbon-12 isotope was chosen
Definition of atomic mass unit:
One atomic mass unit is a mass unit equal to exactly one twelfth (1/12th ) the mass of one atom of Carbon -12.
Molecules of atom:
Atoms of most of the elements do not exist independently. Atoms form molecule. A molecule is a group of two or more atoms, which are chemically bonded together, by attractive forces.
Atomicity:
Number of atoms in the molecules of an element is known as atomicity.
Eg:
Molecules of Argon (Ar), Helium (He) etc are mase of only one atom, so their atomicity is one.
Molecule of oxygen consists of two atoms of oxygen and hence it is known as a diatomic molecule, O2.
