Structure of the atom
Discovery of charged particles in matter
- Goldstein in 1886 discovered presence of new radiations in gas discharged and called them canal ray
- These rays were positively charged radiations
- This led to the discovery of subatomic particle protons
- J. Thomson in 1900 discovered electrons
The structure of atom
Many scientists proposed different model to explain the structure of atom.
1.Dalton’s theory
He proposed that atom was the smallest particle of any substance and an atom was indivisible and indestructible.
Failure of Dalton’s theory:
Discovery of subatomic particles led to the failure of this theory
2.J.J.Thomson’s Model
- He proposed the model of an atom which is similar to that of Christmas pudding.
- The electrons in a sphere of positive charge were like dry fruits in Christmas pudding.We can compare this to watermelon, like seeds of watermelon are studded in the fruit.


Thomson atomic theory:
- An atom consists of a positively charged sphere and the electrons are embedded in it.
- The negative and positive charges are equal in magnitude, so the atom is electrically neutral.
Positive features of Thomson’s model:
This explained atoms are negatively charged.
Why did Thomson model failed?
This could not explain the results of experiments carried by the scientists.
3.Rutherford’s model of an atom:
Experiment conducted by Rutherford(α particles scattering experiment):
Moving alpha particles are made to fall on a thin gold foil.
He selected a gold foil, because he wanted as thin layer as possible. The gold foil he selected was about 1000 atoms thick
α particles :
These are doubly charged Helium ions. They have mass of 4u , hence the fast moving alpha particles have considerable amount of energy.
What he expected:
α particles would be deflected by the subatomic particles in the gold atoms. As α particles are much heavier than protons, he did not expect to see large deflections.

Image courtesy -Google
Observations of the experiment:
- Most of the fast moving α particles passed straight through the gold foil.
- Some of the α particles were deflected by small angles
- Surprisingly one out of every 12000 particles appeared to renounce.
Conclusion:
- Most of the space inside the atom is empty, because most of the α particles passed through the gold foil without getting deflected .
- Very few particles were deflected from their path , indicating that the positive charge of the atom occupies very little space.
- A very small fraction of α particles were deflected by 1800 indicated that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.
- From the above data he calculated the radius of a nucleus is 1015 times less than radius of atom
Nuclear Model of the atom
There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
The electrons revolve around the nucleus in circular path.
The size of nucleus is very small compared to the size of the atom.
Drawback of Rutherford’s model:
- The revolution of electron in a circular orbit is not to be expected stable.
- Any particle in a circular orbit would radiate energy, and loose energy and finally fall into the nucleus. If this were so, the atom should be highly unstable and hence matter would not exist.
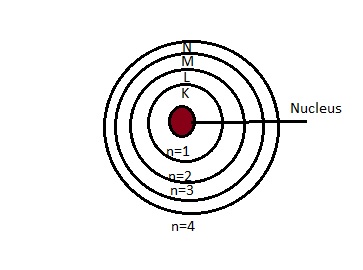
4.Bohr’s Model of an atom:
Postulates:
- Only certain special orbits known as discrete orbits of electrons are allowed inside the atom.
- While revolving in discrete orbits the electrons do not radiate energy.
- These orbits or shells are called energy levels

Neutrons:
- Chadwick discovered neutron. Neutrons do not have charge, and its mass is nearly equal to proton. Neutron are present in the nucleus, of all atoms except hydrogen.
Distribution of electrons:
1.The maximum number of electrons present in a shell is given by the formula 2n2, where n is the orbit number or energy level, and n=1,2,3,4……
2.The maximum number of electrons that can be accommodated in the outermost orbit is 8
3.Electrons are accommodated in the given shell, unless the inner shells are filled.That is shells are filled in stepwise manner.